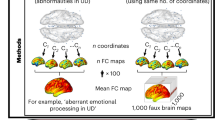
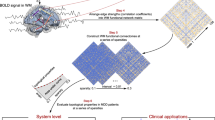
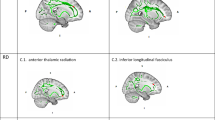
Abstract
Multiple sclerosis (MS), a demyelinating disease that causes focal white matter lesions, is commonly associated with depression. However, it remains unclear whether depression risk is selectively increased by specific white matter lesion locations. Recent work shows that stroke lesions and therapeutic neuromodulation sites that modify depression severity are connected to a common brain circuit, providing an a priori template. Here we assessed whether this circuit is relevant for white matter lesions in MS. In a clinical and radiological database of individuals with MS (n = 281), we estimated the whole-brain connectivity of each person’s white matter lesion locations using a normative connectome database (n = 1,000). Functional connectivity between MS lesion locations and our a priori depression circuit was correlated with depression severity in MS (P = 0.013) and specific to depression versus other MS-related symptoms (P = 0.0058). A data-driven circuit for MS depression showed similar topography to our a priori depression circuit (P = 0.015). The peak of this data-driven MS depression circuit was in the ventral midbrain, including the ventral tegmental area (familywise-error-corrected P < 0.05). These findings lend insight into the localization of MS depression that may help guide targeting for therapeutic brain stimulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$79.00 per year
only $6.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The functional connectivity data employed in this study are available online through the Harvard Dataverse at https://doi.org/10.7910/DVN/ILXIKS. Due to the potentially identifiable nature of MS lesion patterns, these data cannot be shared publicly but may be reviewed upon reasonable request with an institutional data use agreement. The final MS depression circuit is available on our website: https://siddiqi.bwh.harvard.edu/data-code/.
Code availability
The pipeline used to prepare the functional connectivity data is available at https://github.com/bchcohenlab/BIDS_to_CBIG_fMRI_Preproc2016. Statistical analyses were performed in MATLAB R2021b. Custom MATLAB scripts for spatial permutation testing are available on our website: https://siddiqi.bwh.harvard.edu/data-code/.
References
Sadovnick, A. D. et al. Depression and multiple sclerosis. Neurology 46, 628–632 (1996).
Chwastiak, L. et al. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am. J. Psychiatry 159, 1862–1868 (2002).
Arnett, P. A. et al. Depressed mood in multiple sclerosis: relationship to capacity-demanding memory and attentional functioning. Neuropsychology 13, 434–446 (1999).
Feinstein, A., Magalhaes, S., Richard, J. F., Audet, B. & Moore, C. The link between multiple sclerosis and depression. Nat. Rev. Neurol. 10, 507–517 (2014).
Janardhan, V. & Bakshi, R. Quality of life in patients with multiple sclerosis: the impact of fatigue and depression. J. Neurol. Sci. 205, 51–58 (2002).
Siddiqi, S. H. et al. Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease. Nat. Hum. Behav. 5, 1707–1716 (2021).
Pascual-Leone, A., Rubio, B., Pallardó, F. & Catalá, M. D. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet 348, 233–237 (1996).
Siddiqi, S. H., Kording, K. P., Parvizi, J. & Fox, M. D. Causal mapping of human brain function. Nat. Rev. Neurosci. 23, 361–375 (2022).
Safar, L. T., Singhal, T., Feng, N. C., Misasi, E. & Barker, L. in Neuropsychiatry and Behavioral Neurology: Principles and Practice (eds Silbersweig, D. A. et al.) Ch. 27 (McGraw Hill, 2021).
Feinstein, A. et al. Diffusion tensor imaging abnormalities in depressed multiple sclerosis patients. Mult. Scler. J. 16, 189–196 (2010).
Zorzon, M. et al. Depressive symptoms and MRI changes in multiple sclerosis. Eur. J. Neurol. 9, 491–496 (2002).
Bakshi, R. et al. Brain MRI lesions and atrophy are related to depression in multiple sclerosis. Neuroreport 11, 1153–1158 (2000).
Fox, M. D. Mapping symptoms to brain networks with the human connectome. N. Engl. J. Med. 379, 2237–2245 (2018).
Padmanabhan, J. L. et al. A human depression circuit derived from focal brain lesions. Biol. Psychiatry, https://doi.org/10.1016/j.biopsych.2019.07.023 (2019).
Wang, P. et al. White matter functional connectivity in resting-state fMRI: robustness, reliability, and relationships to gray matter. Cereb. Cortex, https://doi.org/10.1093/cercor/bhab181 (2021).
Fox, M. D. et al. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc. Natl Acad. Sci. USA 111, E4367–E4375 (2014).
Gore, J. C. et al. Functional MRI and resting state connectivity in white matter—a mini-review. Magn. Reson. Imaging https://doi.org/10.1016/j.mri.2019.07.017 (2019).
Meier, D. S. et al. Dual-sensitivity multiple sclerosis lesion and CSF segmentation for multichannel 3T brain MRI. J. Neuroimaging 28, 36–47 (2018).
Bakshi, R. et al. Fatigue in multiple sclerosis and its relationship to depression and neurologic disability. Mult. Scler. J. 6, 181–185 (2000).
Siddiqi, S. H. et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am. J. Psychiatry 177, 435–446 (2020).
Masuccio, F. G., Gamberini, G., Calabrese, M. & Solaro, C. Imaging and depression in multiple sclerosis: a historical perspective. Neurol. Sci. 42, 835–845 (2021).
Gold, S. M. & Irwin, M. R. Depression and immunity: inflammation and depressive symptoms in multiple sclerosis. Immunol. Allergy Clin. North Am. 29, 309–320 (2009).
Gaynes, B. N. et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. J. Clin. Psychiatry 75, 29758 (2014).
Shen, X. et al. Repetitive transcranial magnetic stimulation for the treatment of post-stroke depression: a systematic review and meta-analysis of randomized controlled clinical trials. J. Affect. Disord. 211, 65–74 (2017).
Joutsa, J., Horn, A., Hsu, J. & Fox, M. D. Localizing parkinsonism based on focal brain lesions. Brain 141, 2445–2456 (2018).
Ganos, C. et al. A neural network for tics: insights from causal brain lesions and deep brain stimulation. Brain, https://doi.org/10.1093/brain/awac009 (2022).
León Ruiz, M., Sospedra, M., Arce Arce, S., Tejeiro-Martínez, J. & Benito-León, J. Current evidence on the potential therapeutic applications of transcranial magnetic stimulation in multiple sclerosis: a systematic review of the literature. Neurología 37, 199–215 (2022).
Nestler, E. J. & Carlezon, W. A. Jr The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 59, 1151–1159 (2006).
Heitmann, H. et al. Fatigue, depression, and pain in multiple sclerosis: how neuroinflammation translates into dysfunctional reward processing and anhedonic symptoms. Mult. Scler. J. https://doi.org/1352458520972279 (2020).
Pardini, M., Capello, E., Krueger, F., Mancardi, G. & Uccelli, A. Reward responsiveness and fatigue in multiple sclerosis. Mult. Scler. J. 19, 233–240 (2013).
Felger, J. C. in Inflammation-Associated Depression: Evidence, Mechanisms and Implications (eds Dantzer, R. & Capuron, L.) 199–219 (Springer, 2017).
Marcus, R. N. et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J. Clin. Psychopharmacol. 28, 156–165 (2008).
Suppes, T. et al. Lurasidone for the treatment of major depressive disorder with mixed features: a randomized, double-blind, placebo-controlled study. Am. J. Psychiatry 173, 400–407 (2016).
Loebel, A. et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am. J. Psychiatry 171, 160–168 (2014).
Rush, A. J. et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. New Engl. J. Med. 354, 1231–1242 (2006).
Bodkin, J. A. & Amsterdam, J. D. Transdermal selegiline in major depression: a double-blind, placebo-controlled, parallel-group study in outpatients. Am. J. Psychiatry 159, 1869–1875 (2002).
Amtmann, D. et al. A comparison of multiple patient reported outcome measures in identifying major depressive disorder in people with multiple sclerosis. J. Psychosom. Res. 79, 550–557 (2015).
Chan, C. K., Tian, F., Pimentel Maldonado, D., Mowry, E. M. & Fitzgerald, K. C. Depression in multiple sclerosis across the adult lifespan. Mult. Scler. J. 27, 1771–1780 (2021).
Miller, D. M. et al. Validating Neuro-QoL short forms and targeted scales with people who have multiple sclerosis. Mult. Scler. J. 22, 830–841 (2016).
Healy, B. C. et al. Assessment of computer adaptive testing version of the Neuro-QOL for people with multiple sclerosis. Mult. Scler. J. 25, 1791–1799 (2019).
Medina, L. D., Torres, S., Alvarez, E., Valdez, B. & Nair, K. V. Patient-reported outcomes in multiple sclerosis: validation of the quality of life in neurological disorders (Neuro-QoL™) short forms. Mult. Scler. J. 5, 2055217319885986 (2019).
Hua, L. H., Hersh, C. M., Tian, F., Mowry, E. M. & Fitzgerald, K. C. Clinical characteristics of a large multi-center cohort of people with multiple sclerosis over age 60. Mult. Scler. Relat. Disord. 47, 102637 (2021).
Kletenik, I. et al. Subjective cognitive concern in multiple sclerosis is associated with reduced thalamic and cortical gray matter volumes. Mult. Scler. J. 5, 2055217319827618 (2019).
Fox, M. D., Liu, H. & Pascual-Leone, A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage 66, 151–160 (2013).
Kelley, K. & Preacher, K. J. On effect size. Psychol. Methods 17, 137–152 (2012).
Polman, C. H. et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 69, 292–302 (2011).
Kurtzke, J. F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33, 1444–1444 (1983).
Klein, S., Staring, M., Murphy, K., Viergever, M. A. & Pluim, J. P. elastix: a toolbox for intensity-based medical image registration. IEEE Trans. Med. Imaging 29, 196–205 (2010).
Foster, J. C., Nan, B., Shen, L., Kaciroti, N. & Taylor, J. M. Permutation testing for treatment–covariate interactions and subgroup identification. Stat. Biosci. 8, 77–98 (2016).
Cremers, H. R., Wager, T. D. & Yarkoni, T. The relation between statistical power and inference in fMRI. PLoS ONE 12, e0184923 (2017).
Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W. & Smith, S. M. FSL. Neuroimage 62, 782–790 (2012).
Slotnick, S. D. Cluster success: fMRI inferences for spatial extent have acceptable false-positive rates. Cogn. Neurosci. 8, 150–155 (2017).
Levinson, S. et al. A structural connectivity atlas of limbic brainstem nuclei. Preprint at bioRxiv https://doi.org/10.1101/2022.08.10.503282 (2022).
Horn, A. et al. Lead-DBS v2: towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage 184, 293–316 (2019).
Acknowledgements
The authors thank all research participants, funding bodies, allied health staff and other research staff that made this work possible. We thank M. Polgar-Turcsanyi for database management. The SysteMS study was funded by Verily Life Sciences. The present analysis was supported by the Brain & Behavior Research Foundation (S.H.S.), the Baszucki Family Foundation (S.H.S. and M.D.F.), and the National Institute of Mental Health (grant no. K23MH121657 to S.H.S.; grant numbers R01MH113929 and R01MH115949 to M.D.F.). The funders were not directly involved in the conceptualization, design, analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conception and design of study were by S.H.S., I.K., R.B., C.R.G.G. and M.D.F. Lesion network mapping and statistical analyses were by S.H.S. and I.K. Preprocessing and preparation of data for analysis were by M.C.A., M.C., S.K. and M.P. Data collection was by R.B., C.R.G.G., M.C., T.C. and B.I.G. Writing of the manuscript was by S.H.S., I.K. and M.D.F. with input from all authors.
Corresponding author
Ethics declarations
Competing interests
S.H.S. and M.D.F. are scientific consultants for Magnus Medical. S.H.S. is a clinical consultant for Acacia Mental Health, Kaizen Brain Center and Boston Precision Neurotherapeutics. S.H.S. and M.D.F. have jointly received investigator-initiated research funding from Neuronetics. S.H.S. has served as a speaker for Brainsway and PsychU.org (unbranded, sponsored by Otsuka). S.H.S. and M.D.F. independently own intellectual property involving the use of functional connectivity to target TMS. R.B. has received consulting fees from Bristol Myers Squibb and EMD Serono and research support from Bristol Myers Squibb, EMD Serono and Novartis. C.R.G.G. has received research funding from Sanofi, the National Multiple Sclerosis Society, the International Progressive Multiple Sclerosis Alliance, the US Office for Naval Research, NIH, the Focused Ultrasound Foundation, Bristol Myers Squibb/Celgene as well as travel support from Roche Pharmaceuticals, and owns stock in Roche, Novartis, GSK, Alnylam, Protalix Biotherapeutics, Arrowhead Pharmaceuticals, Cocrystal Pharma and Sangamo Therapeutics. None of these entities was involved in the present work. The remaining authors have no conflicts of interest to declare.
Peer review
Peer review information
Nature Mental Health thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2 and Tables 1–3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Siddiqi, S.H., Kletenik, I., Anderson, M.C. et al. Lesion network localization of depression in multiple sclerosis. Nat. Mental Health 1, 36–44 (2023). https://doi.org/10.1038/s44220-022-00002-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44220-022-00002-y